Their choices change,
but one choice is clear:
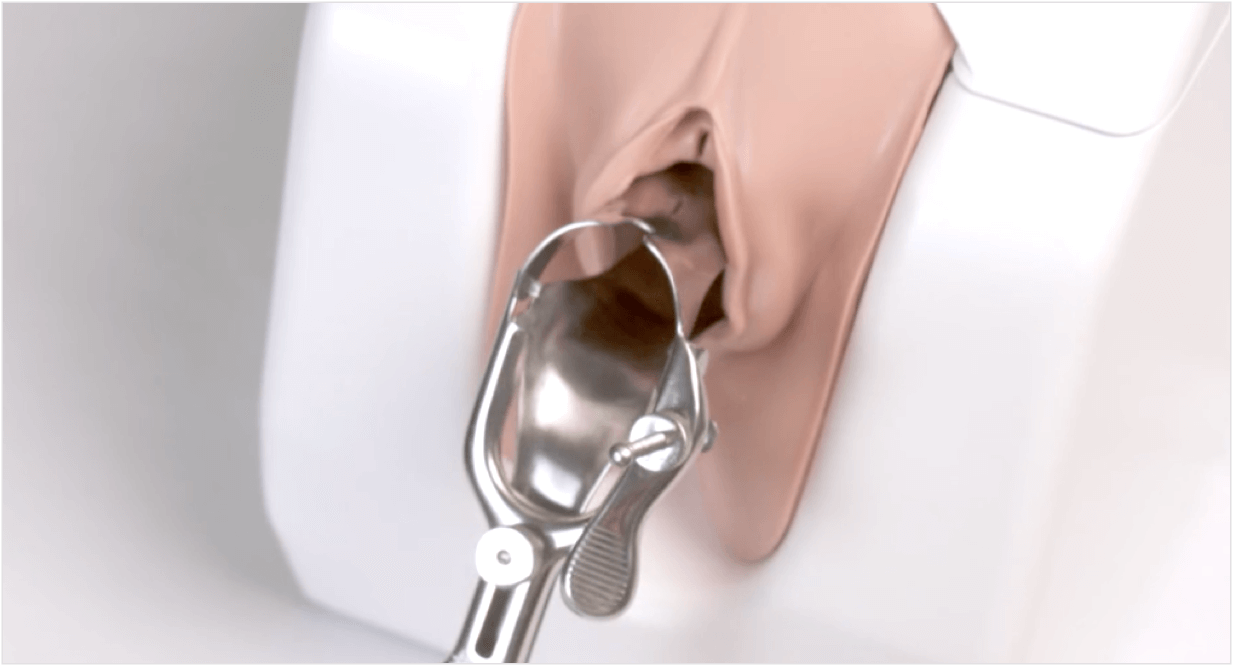
Hormone-Free Paragard®1
From running the school social scene to running a startup
Offer your patients a birth control that won’t interfere with them or their future choices. Paragard is ready for any stage they enter next.
Ordering and
Reimbursement
Placement and Removal
Training