The most effective hormone-free birth control1*
Paragard® IUS is >99% Effective1
Percentage of women who will not become pregnant within 1 year (typical use)‡




























Over 99% effective
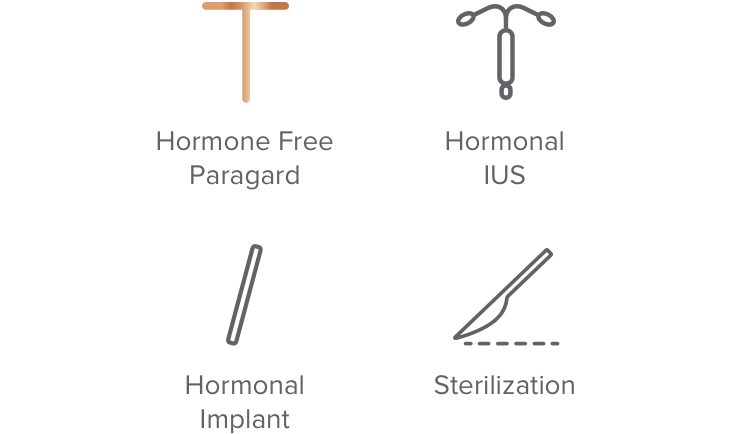
91% – 99% effective
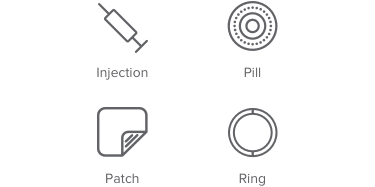
74% – 90% effective
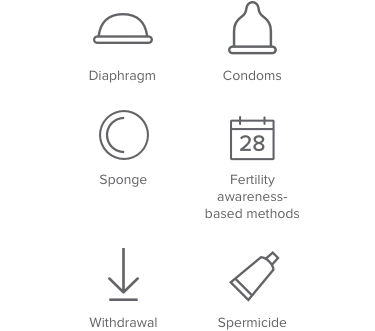
Less than 73% effective

Over 99% effective

74% – 99% effective
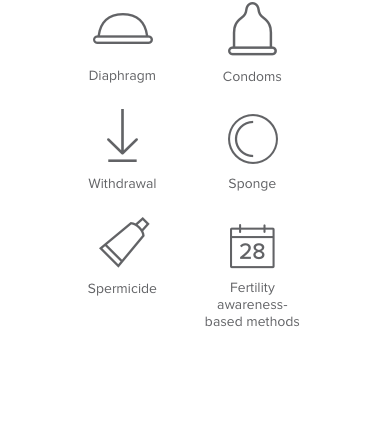
Less than 73% effective

Over 99% effective
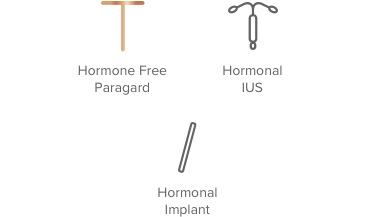
91% – 99% effective
Trussell J. Contraceptive failure in the United States. Contraception 2011 May;83(5):397-404.
Paragard does not protect against HIV/AIDS or other sexually transmitted infections.

