Safety Considerations

Safety Profile
Paragard should not be placed when one or more of the following conditions exist:
- Pregnancy or suspicion of pregnancy
- Abnormalities of the uterus resulting in distortion of the uterine cavity
- Acute pelvic inflammatory disease (PID)
- Postpartum endometritis or postabortal endometritis in the past 3 months
- Known or suspected uterine or cervical malignancy
- Uterine bleeding of unknown etiology
- Untreated acute cervicitis or vaginitis or other lower genital tract infection
- Conditions associated with increased susceptibility to pelvic infections
- Wilson’s disease
- A previously placed IUD or IUS that has not been removed
- Hypersensitivity to any component of Paragard including copper or any of
the trace elements present in the copper component of Paragard
Safety Profile
Paragard should not be placed when one or more of the following conditions exist:
- Pregnancy or suspicion of pregnancy
- Abnormalities of the uterus resulting in distortion of the uterine cavity
- Acute pelvic inflammatory disease (PID)
- Postpartum endometritis or postabortal endometritis in the past 3 months
- Known or suspected uterine or cervical malignancy
- Uterine bleeding of unknown etiology
- Untreated acute cervicitis or vaginitis or other lower genital tract infection
- Conditions associated with increased susceptibility to pelvic infections
- Wilson’s disease
- A previously placed IUD or IUS that has not been removed
- Hypersensitivity to any component of Paragard including copper or any of the trace elements present in the copper component of Paragard

Warnings & Precautions
- Ectopic pregnancy
- Risks with Intrauterine Pregnancy
- Sepsis
- Pelvic Inflammatory Disease (PID) and Endometritis
- Embedment
- Perforation
- Expulsion
- Wilson’s disease
- Bleeding pattern alterations
- MRI safe under certain conditions
- Medical diathermy may cause health effects
Adverse Reactions
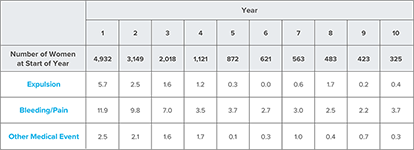
Summary of rates* (no. per 100 subjects) by year for adverse reactions causing discontinuation
*Rates were calculated by weighting the annual rates by the number of subjects starting each year for each of the US Composite Study (3536 subjects) and the World Health Organization (1396 subjects) trials.
The most serious adverse events associated with Paragard include:
- Ectopic pregnancy
- Intrauterine pregnancy
- Septic abortion
- Group A Streptococcal Sepsis (GAS)
- Pelvic Inflammatory Disease (PID) and Endometritis
- Embedment
- Perforation
- Expulsion
- Bleeding pattern alterations
The following adverse events have also been observed:
- Anemia
- Backache
- Dysmenorrhea
- Dyspareunia
- Complete or partial expulsion
- Prolonged menstrual flow
- Menstrual spotting
- Pain and cramping
- Vaginitis