Strong continuation rates and high patient satisfaction1,2
Paragard® IUS has strong continuation rates and high patient satisfaction
Paragard continuation rates are equal to or greater than those of other LARCs1,2*†
Contraceptive continuation rates at 12 months among women ≥26 years of age (n=2,956)
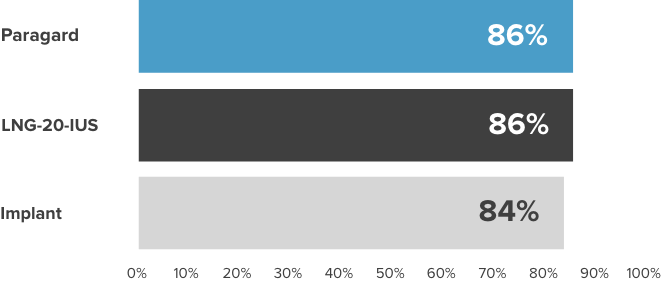
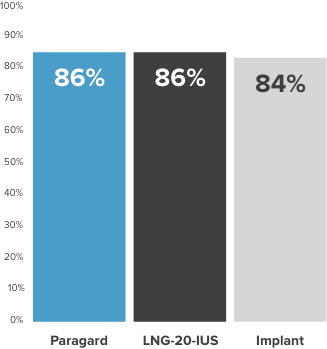
LARC=Long-Acting Reversible Contraceptive; LNG-20-IUS=Ievonorgestrel 20 mcg/day intrauterine system.
*From the Contraceptive CHOICE Project (N=7,472). The (n=2,956) is a subset of the total (N=7,472). A prospective cohort study to determine the continuation rates and satisfaction levels with many reversible methods of contraception. To be included in this analysis, study participants had to initiate their chosen contraceptive within 3 months of CHOICE enrollment and reach the time point for a 12-month follow-up telephone survey. Follow-up telephone interviews were conducted at 3, 6, and 12 months. A “continuer” was a participant who reported using her baseline contraceptive without stopping temporarily for ≥1 month.
†Chart does not compare the safety or efficacy of these methods.
Paragard can be used by most females of reproductive age, including nulliparous and adolescent patients3
94.4% had first-attempt placement success for Paragard
in adolescents and young women (13-24 years)4‡

93% of women using Paragard reported satisfaction
at 3 and 6 months postplacement1§

87% were likely to recommend Paragard
to a friend or family member5¶
‡Total study N=1,177; Paragard users n=269. No significant difference in first-attempt success was found by IUS type.
§According to a study of 5,011 LARC users (826 using Paragard), who were asked to report changes in bleeding and cramping since their LARC was placed.
Paragard allows patients to keep their normal cycle3
In a survey, more women reported experiencing no significant changes in their natural menstrual cycle than those who did5¶
A different study showed that over the first 6 months using Paragard, women reported6:

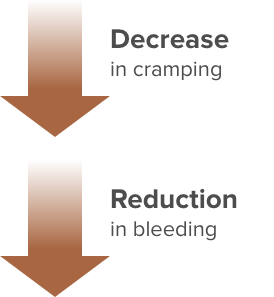
Bleeding significantly decreased (∼23%) from an estimated Pictorial Blood Loss Assessment Chart (PBAC)=195 at one month post-insertion to PBAC=151 at 6 months. IUD satisfaction improved over time, increasing from between “Neutral and Satisfied” to “Satisfied,” and cramping decreased from “biweekly and weekly” to “once or twice a month” over the 6-month study.
Say yes to periods
According to a Sermo survey of 1,004 women:

of Paragard users preferred to keep their period5¶
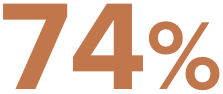
liked having a menstrual cycle as a reminder they were not pregnant5¶
¶Based on an April 2023 web-based survey of women aged 18-50 (n=1,004) who currently use Paragard birth control and have for at least 1 year 48% reported no significant changes, 46% reported changes, and 6% did not remember. At first, periods may become heavier and longer with spotting in between.
Bleeding Profile

Paragard users may experience heavier and longer periods with spotting in between; usually this decreases over time3
Paragard will not interfere with her menstrual cycle or stop ovulation3
Patients should be advised to call you if spotting persists or their menstrual flow continues to be heavy or long. If a woman misses her period, she must be promptly evaluated for pregnancy.


