Step-by-step with an improved placement process1
Placement & Removal Training Videos
Watch our step-by-step videos showing different methods of placing Paragard®.
Welcome to the Placement Training Video for Paragard (intrauterine copper contraceptive) with single-hand inserter. This video will guide you through the preparation, loading, placement, and removal of Paragard. Listen to the Important Safety Information at the end of this video and visit hcp.paragard.com for the full Prescribing Information. Before considering use of Paragard, make sure the patient is an appropriate candidate and exclude pregnancy.
Let’s prepare for Paragard placement.
Note: The inserter provided with Paragard and the Insertion Procedure described in this video are not applicable for immediate insertion after childbirth. For immediate insertion post childbirth, remove the inserter and Paragard from the tray, move the button forward and then completely back to release the Paragard unit from the inserter and insert Paragard.
Use strict aseptic technique throughout preparation.
To begin, prepare the placement tools and consider the use of an analgesic. Tools include speculum, cotton swab, sterile tenaculum, sterile uterine sound, scissors and sterile forceps.
Establish the size and position of the uterus by performing a bi-manual examination.
Then, insert a speculum and use a cotton swab with an antiseptic solution to cleanse the cervix and vagina.
Apply a tenaculum to the cervix and use gentle traction to align the cervical canal with the uterine cavity.
Gently insert a sterile uterine sound to measure the depth of the uterine cavity. The uterus should sound to a depth of 6 to 9 cm except when placing Paragard immediately postabortion or immediately postpartum.
It is important to note that placement of Paragard may be associated with pain and/or bleeding or vasovagal reactions such as syncope, bradycardia, or seizure, especially in patients with a predisposition to these symptoms. Placement of Paragard into a uterine cavity measuring less than 6 cm may increase the incidence of expulsion, bleeding, pain, and perforation.
If cervical stenosis is encountered, avoid undue force. Dilators and analgesia or local anesthesia may be helpful in this situation.
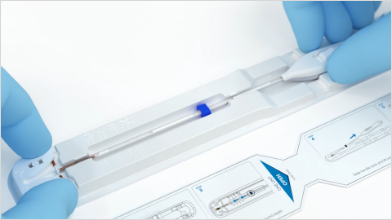
After preparation, it’s time to load Paragard. When loading Paragard, use strict aseptic techniques throughout the procedure.
Place the package containing Paragard (face-up) and instructions on a sterile field and open pouch from the handle end where arrow says “OPEN”. Remove the clear cover from the tray. The inserter should remain in the tray.
Confirm that the top of the button is located at the starting line on the handle prior to loading Paragard.
Using sterile gloves, place one hand on the distal end of the tray and the other on the inserter handle.
Slide the handle completely forward so that the Paragard advances into the Loading Tip folding the T-arms of Paragard against the stem.
Once the T-arms are folded against the stem, slide the button on the handle completely forward to advance the insertion tube over the tips of the T-arms.
Only the tips of the T-arms should be in the insertion tube. Don’t advance beyond the copper collars.
After loading, be sure not to leave the horizontal arms of Paragard bent for more than 5 minutes, as this may cause the arms not to open properly.
Now that Paragard is fully loaded in the insertion tube, the next step is to adjust the blue flange to the premeasured uterine depth.
Grasp the insertion tube and use the ruler on the tray to adjust the blue flange so that the distance from the top of the loaded Paragard to the top of the blue flange is the same as the premeasured uterine depth.
The top of Paragard should align with the zero marking at the loading end while setting the blue flange.
The flange may be sticky with first movement attempt.
Ensure the button remains in the forward position.
To remove the inserter from the tray, gently lift the handle out of the tray, then gently slide the inserter back and lift out of the tray.
Make sure both T-arms are captured in the tube and that the button is completely forward.
Upon removal from the tray, verify and rotate the blue flange as needed so that the horizontal arms of Paragard and the long axis of the blue flange and handle lie in the same horizontal plane to ensure the arms open in the proper direction. Now, Paragard is prepared for uterine placement.
Once Paragard is properly loaded, the next step is placement.
To begin, apply gentle traction to the tenaculum to orient the uterus in an axial position.
While holding the button forward, pass the loaded insertion tube through the cervical canal until Paragard just touches the fundus of the uterus. This will ensure placement of Paragard is in the highest possible position within the uterus. The blue flange should be at the cervix in the horizontal plane. The button should remain in the forward position.
Hold the inserter at the fundus while you slide the button all the way back. Do not stop at the starting line as it is not used for deployment.
This will release the T-arms of Paragard high in the uterine fundus.
Gently and slowly withdraw the inserter from the uterus and cervical canal.
After placement, only the threads should be visible protruding from the cervix. Trim the threads so that 3 to 4 cm protrude into the vagina.
Measure the protrusion of the threads and record the length, date of device placement, and Paragard lot number in the patient’s chart.
If you are concerned that Paragard is not in the correct position, check the placement of the device. This can be done with ultrasound, if necessary.
If Paragard is not positioned completely within the uterus, remove it and replace with a new Paragard. Do not reinsert an expelled or partially expelled Paragard.
As a follow up to placement, examine the patient after her first menses to confirm that Paragard is still in place.
You should be able to see or feel only the threads. The length of the visible threads may change with time, but no action is needed unless you suspect partial expulsion, perforation, pregnancy, or breakage.
If you are unable to find the threads in the vagina, check that Paragard is still in the uterus. The threads can retract into the uterus or break, or Paragard can break, perforate the uterus, or be expelled. Gentle probing of the cavity, x-ray, or sonography may be required to locate Paragard.
If Paragard has been partially expelled or has perforated the uterus, remove the device. Do not reinsert a used Paragard.
Paragard must be removed no later than 10 years after placement, but may be removed at any time prior to this. A new Paragard can be placed at the time of removal if continued contraceptive protection is desired.
To remove Paragard, begin by visualizing the cervix using a speculum. Remove Paragard with forceps, pulling gently on the exposed threads.
The arms of Paragard will fold upwards as it is withdrawn from the uterus. Once removed, make sure Paragard is intact.
Breakage or embedment of Paragard in the myometrium can make removal difficult. Analgesia, paracervical anesthesia, cervical dilation, a grasping instrument like alligator forceps, or hysteroscopy may assist in removing an embedded Paragard.
Removal of Paragard may be associated with some pain, bleeding, or vasovagal reactions like syncope, bradycardia, and seizures, especially in patients with a predisposition to these conditions.
If you have any questions about the product or procedure, please contact us at 1-877-727-2427 or visit hcp.paragard.com.
Continue watching for a review of the Indications, Usage, and Important Safety Information for Paragard. Refer to the full Prescribing Information for more detail regarding preparations for placement, warnings, contraindications, adverse reactions, and other important information regarding Paragard.
INDICATIONS AND USAGE
Paragard is a copper-containing intrauterine system (IUS) indicated for prevention of pregnancy in females of reproductive potential for up to 10 years.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
- The use of Paragard is contraindicated when one or more of the following conditions exist:
- Pregnancy or suspicion of pregnancy, abnormalities of the uterus resulting in distortion of the uterine cavity, acute pelvic inflammatory disease (PID), postpartum or postabortal endometritis in the past 3 months, known or suspected uterine or cervical malignancy, uterine bleeding of unknown etiology, untreated acute cervicitis or vaginitis or other lower genital tract infection, conditions associated with increased susceptibility to pelvic infections, Wilson’s disease, a previously placed IUS that has not been removed, hypersensitivity to any component of Paragard including to copper or any of the trace elements present in the copper component of Paragard.
WARNINGS AND PRECAUTIONS
- Ectopic Pregnancy: Evaluate for possible ectopic pregnancy in any female who becomes pregnant while using Paragard.
- Intrauterine Pregnancy: Failure to remove Paragard increases the risk of miscarriage, sepsis, premature labor, and premature delivery.
- Sepsis: Severe infection or sepsis, including Group A Streptococcal Sepsis (GAS), have been reported following insertion of IUSs, including Paragard.
- Pelvic Inflammatory Disease and Endometritis: Remove Paragard in cases of recurrent PID or endometritis, or if an acute pelvic infection is severe or does not respond to treatment.
- Embedment: Partial penetration or embedment of Paragard in the myometrium can make removal difficult; surgical removal may be necessary. Breakage of an embedded Paragard during non-surgical removal has been reported.
- Perforation: Partial or total perforation of the uterine wall or cervix may reduce contraceptive efficacy and result in pregnancy. Delayed detection or removal of Paragard may result in migration outside the uterine cavity, adhesions, peritonitis, intestinal penetration, intestinal obstruction, abscesses and/or damage to adjacent organs. Increased risk when the uterus is fixed, retroverted or not completely involuted during the postpartum period. If perforation does occur, locate and remove Paragard promptly.
- Expulsion: Partial or complete expulsion of Paragard has been reported, resulting in the loss of contraceptive protection. The risk of expulsion may be increased when the uterus is not completely involuted at the time of insertion. Remove a partially expelled Paragard.
- Wilson’s Disease: Paragard may exacerbate Wilson’s disease.
- Bleeding Pattern Alterations: Paragard can alter the bleeding pattern and result in heavier and longer menstrual cycles with intermenstrual spotting.
- Magnetic Resonance Imaging (MRI) Safety Information: Non-clinical testing has demonstrated that Paragard is MR Conditional.
- Medical Diathermy: Avoid using high medical RF transmitter devices in females with Paragard.
ADVERSE REACTIONS
- Adverse reactions reported in clinical trials include anemia, backache, dysmenorrhea, dyspareunia, expulsion (complete or partial), prolonged menstrual flow, menstrual spotting, pain and cramping, and vaginitis.
Please see full Prescribing Information at hcp.paragard.com
Welcome to the Placement Training Video for Paragard (intrauterine copper contraceptive). This video will guide you through the preparation, loading, placement, and removal of Paragard.
Listen to the Important Safety Information at the end of this video and visit hcp.paragard.com for the full Prescribing Information. Before considering use of Paragard, make sure the patient is an appropriate candidate and exclude pregnancy. Let’s prepare for Paragard placement.
To begin, prepare the placement tools and consider the use of an analgesic. Tools include speculum, cotton swab, sterile tenaculum, sterile uterine sound, sterile scissors, and sterile forceps.
Establish the size and position of the uterus by performing a bi-manual examination. Then, insert a speculum and use a cotton swab with an antiseptic solution to cleanse the cervix and vagina. Apply a tenaculum to the cervix and use gentle traction to align the cervical canal with the uterine cavity.
Gently insert a sterile uterine sound to measure the depth of the uterine cavity. The uterus should sound to a depth of 6 to 9 cm except when placing Paragard immediately postabortion or immediately postpartum. It is important to note that placement of Paragard may be associated with pain and bleeding or vasovagal reactions such as syncope, bradycardia, or seizure, especially in patients with a predisposition to these symptoms. Placement of Paragard into a uterine cavity measuring less than 6 cm may increase the incidence of expulsion, bleeding, pain, and perforation. If cervical stenosis is encountered, avoid undue force. Dilators and analgesia or local anesthesia may be helpful in this situation. After preparation, it’s time to load Paragard. There are two ways to load this device.
Paragard can be loaded outside of the package with sterile gloves, or, if sterile gloves are not available, you can load Paragard inside of the package. Paragard should not be loaded more than 5 minutes before being placed into the uterus. When loading Paragard outside of the package, use strict aseptic techniques throughout the procedure. Start by placing the Paragard package, face up, on a clean surface. Then, open the package from the bottom end where the arrow says “open.” Using sterile gloves, bend the T-Arms of Paragard by folding the two horizontal arms down against the stem. Then, slightly withdraw the insertion tube, push the arms down along the stem and slide the insertion tube over the tips of the T-Arms. At this point, only the tips of the T-Arms should be in the insertion tube, do not advance beyond the copper collars. After loading, be sure not to leave the horizontal arms of Paragard bent for more than 5 minutes, this may cause the arms not to open properly. Next, take the solid white rod and insert it into the bottom of the insertion tube until it touches the bottom of Paragard.
Now that Paragard is fully loaded in the insertion tube, the next step is to adjust the flange to the same depth that you measured during uterine sounding. Grasp the insertion tube and use the ruler on the sterile card to adjust the blue flange so that the distance from the top of the loaded Paragard to the top of the blue flange is the same as the uterine depth measured earlier. Rotate the blue flange so that the horizontal arms of Paragard and the long axis of the blue flange lie in the same horizontal plane to ensure the arms open-up in the correct direction. Now, Paragard is prepared for uterine placement.
If sterile gloves are not available, and you are loading Paragard in the sterile package, start by laying the package, facing up, on a clean surface. Open the package from the bottom end where the arrow says “open.” Pull the solid white rod out and then put it back in the package, laying it carefully alongside the insertion tube, ensuring the distal end of the rod remains sterile. Then, place your thumb and index finger on the outside of the package, on top of the ends of the horizontal arms. Use your other hand to push the insertion tube against the arms of Paragard and bend the T-Arms downward. Continue to bring your thumb and index finger closer together on the outside of the package to bend the arms until they are alongside the stem. Use your other hand to withdraw the insertion tube slightly so that it can be pushed and rotated over the tips of the T-Arms. It is important that only the tips of the T-Arms are in the insertion tube. Don’t advance beyond the copper collars. Insert the solid white rod into the bottom of the insertion tube until it touches the bottom of the Paragard. After loading, be sure not to leave the horizontal arms of Paragard bent for more than 5 minutes, this may cause the arms not to open properly. Now that Paragard is fully loaded in the insertion tube, the next step is to adjust the flange to the same depth that you measured during uterine sounding. Grasp the insertion tube at the open end of the package and use the ruler on the sterile card to adjust the blue flange so that the distance from the top of the loaded Paragard to the top of the blue flange is the same as the uterine depth measured earlier. Rotate the blue flange so that the horizontal arms of Paragard and the long axis of the blue flange lie in the same horizontal plane to ensure the arms open-up in the correct direction. Now, Paragard is prepared for uterine placement.
Once Paragard is properly loaded, the next step is placement. To begin, orient the uterus in an axial position and apply gentle traction to the tenaculum. Then, pass the loaded insertion tube through the cervical canal until Paragard just touches the fundus of the uterus. The blue flange should be at the cervix in the horizontal plane. Release the arms of Paragard by holding the solid white rod steady and withdrawing the insertion tube no more than one centimeter. This will release the arms of Paragard high in the uterine fundus. To make sure that the device has been placed high in the fundus, gently and carefully move the insertion tube upward toward the fundus until slight resistance is felt. It is important not to use the white rod as a plunger to push or insert Paragard. Now, hold the insertion tube steady and withdraw the solid white rod. Don’t remove the solid white rod and insertion tube at the same time as this could accidentally pull the device’s threads. Gently and slowly withdraw the insertion tube from the cervical canal. After placement, only the threads should be visible protruding from the cervix. Trim the threads so that 3 to 4 cm protrude into the vagina. Measure the protrusion of the threads and record the length, date of device placement, and Paragard lot number in the patient’s chart. If you are concerned that Paragard is not in the correct position, check the placement of the device. This can be done with ultrasound, if necessary. If Paragard is not positioned completely within the uterus, remove it, and replace with a new Paragard. Do not reinsert an expelled or partially expelled Paragard. As a follow up to placement, examine the patient after her first menses to confirm that Paragard is still in place. You should be able to see or feel only the threads. The length of the visible threads may change at that time, but no action is needed unless you suspect partial expulsion, perforation, pregnancy, or breakage. If you are unable to find the threads in the vagina, check that Paragard is still in the uterus. The threads can retract into the uterus or break, or Paragard can break, perforate the uterus, or be expelled. Gentle probing of the cavity, x-ray, or sonography may be required to locate Paragard. If Paragard has been partially expelled or has perforated the uterus, remove the device. Do not reinsert a used Paragard.
Paragard must be removed no later than 10 years after placement, but may be removed at any time prior to this. A new Paragard can be placed at the time of removal if continued contraceptive protection is desired. To remove Paragard, begin by visualizing the cervix using a speculum. Remove Paragard with forceps, pulling gently on the exposed threads. The arms of Paragard will fold upwards as it is withdrawn from the uterus. Once removed, make sure Paragard is intact. Breakage or embedment of Paragard in the myometrium can make removal difficult. Analgesia, paracervical anesthesia, cervical dilation, a grasping instrument like alligator forceps, or hysteroscopy may assist in removing an embedded Paragard. Removal of Paragard may be associated with some pain, bleeding, or vasovagal reactions like syncope, bradycardia, and seizures, especially in patients with a predisposition to these conditions.
If you have any questions about the product or procedure, please contact us at 1-877-727-2427 or visit hcp.paragard.com. Continue watching for a review of the Indications, Usage, and Important Safety Information for Paragard. Refer to the full Prescribing Information for more detail regarding preparations for placement, warnings, contraindications, adverse reactions, and other important information regarding Paragard.
Paragard placement is improved with single-hand insertion with built-in loading tip1
of HCPs achieved successful Paragard placement with this inserter1
- 91% with first attempt
- 99% with second attempt
Request Placement
Training Materials
Get your training kit and demo unit for Paragard
Clinical Specialist
Education Sessions

Join workshops on a variety of Paragard topics led by our licensed Clinical Specialist Team










