About Paragard®
The IUS that won’t interfere with your patient
or their future choices.

Long-Lasting Protection With Continuous Efficacy That Lasts up to 10 Years1
Paragard is one of the most effective birth control options available*†

#1 Hormone-Free IUS
Talk to your patients about hormone-free options
Did you know?
- 71% of women who use or are planning to use birth control have concerns about hormones3‡
- 1 in 4 Paragard users wants a hormone-free option because they want to continue to have a monthly period4
Immediately Reversible Whenever They Decide1
Paragard can be removed at year 1, year 10, or anytime in between, and patients can start trying to conceive the same day it’s removed§

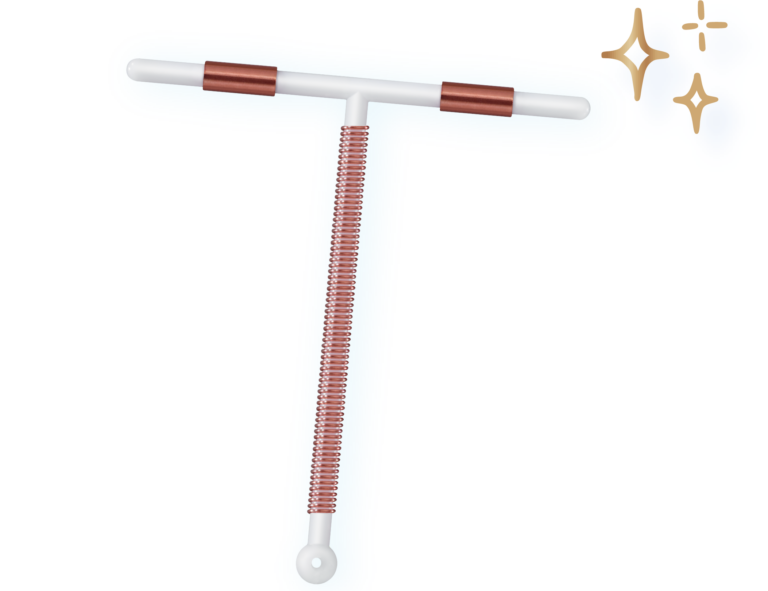
Now With Improved Placement Process
Place Paragard efficiently with a single-hand inserter and built-in loading tip1

Allows Patients to Keep Their Normal Cycle1
In a survey, more women reported experiencing no significant changes in their natural menstrual cycle than those who did.4¶

High Patient Satisfaction
93% of women using Paragard have reported satisfaction at 3 and 6 months post-placement4II
How Does Paragard
Work?
Paragard uses 1 simple active ingredient—copper—to prevent pregnancy. Copper enhances contraceptive efficacy by
interfering with sperm transport and fertilization, possibly
preventing implantation.1†
LARC=Long-Acting Reversible Contraceptive.
*Excluding sterilization.
†Paragard is over 99% effective.
‡Based on an August 2021 web-based survey of women aged 18-45 (n=1,063) who currently use birth control or plan to use birth control in the next 12 months.
§Paragard must be removed by a healthcare provider.
¶Based on an April 2023 web-based survey of women aged 18-50 (n=1,004) who currently use Paragard birth control and have for at least 1 year, 48% reported no significant changes, 46% reported changes, and 6% did not remember. At first, periods may become heavier and longer with spotting in between.
||According to a study of 5,011 LARC users (826 using Paragard), who were asked to report changes in bleeding and cramping since their LARC was placed.

